|
Word Gems
exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
|
Rutherford’s atomic model speaks of a “nucleus.” Unlike the diffused “plum pudding” view, the atom’s positive charge, and almost all of its mass, is concentrated in an extremely small central region.
|
return to "Quantum Mechanics" main-page

Ernest Rutherford (1871 - 1937)
Wikipedia:
Ernest Rutherford, 1st Baron Rutherford of Nelson, OM, FRS, HonFRSE (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics. Encyclopædia Britannica considers him to be the greatest experimentalist since Michael Faraday (1791–1867). Apart from his work in his homeland, he spent a substantial amount of his career abroad, in both Canada and the United Kingdom.
In early work, Rutherford discovered the concept of radioactive half-life, the radioactive element radon, and differentiated and named alpha and beta radiation. This work was performed at McGill University in Montreal, Quebec, Canada. It is the basis for the Nobel Prize in Chemistry he was awarded in 1908 "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances", for which he was the first Oceanian Nobel laureate, and the first to perform the awarded work in Canada. In 1904, he was elected as a member to the American Philosophical Society.
Rutherford moved in 1907 to the Victoria University of Manchester (today University of Manchester) in the UK, where he and Thomas Royds proved that alpha radiation is helium nuclei. Rutherford performed his most famous work after he became a Nobel laureate. In 1911, although he could not prove that it was positive or negative, he theorized that atoms have their charge concentrated in a very small nucleus, and thereby pioneered the Rutherford model of the atom, through his discovery and interpretation of Rutherford scattering by the gold foil experiment of Hans Geiger and Ernest Marsden. He performed the first artificially induced nuclear reaction in 1917 in experiments where nitrogen nuclei were bombarded with alpha particles. As a result, he discovered the emission of a subatomic particle which, in 1919, he called the "hydrogen atom" but, in 1920, he more accurately named the proton.
Rutherford became Director of the Cavendish Laboratory at the University of Cambridge in 1919. Under his leadership the neutron was discovered by James Chadwick in 1932 and in the same year the first experiment to split the nucleus in a fully controlled manner was performed by students working under his direction, John Cockcroft and Ernest Walton. After his death in 1937, he was buried in Westminster Abbey near Sir Isaac Newton. The chemical element rutherfordium (element 104) was named after him in 1997.
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
[Rutherford recalling in 1936 the discovery of the nucleus in 1909, when some alpha particles were observed instead of travelling through a very thin gold foil were seen to rebound backward, as if striking something much more massive than the particles themselves. He won the Nobel Prize in Chemistry for this discovery.]
from reference.com
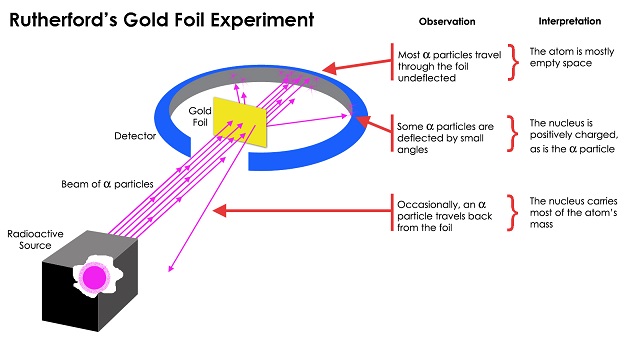
Rutherford’s atomic theory was that an atom had a central positive nucleus with negative electrons orbiting it. He developed this theory with his [1909] gold foil experiment. [This] experiment involved a particle emitter, a round detecting screen with a slit in it and a slip of gold foil in the middle. The detecting screen had zinc sulfide in it to allow Rutherford to detect the presence of particles after they passed through the filtering gold foil.
Through this experiment, Rutherford determined that the vast majority of the particles he fired at the gold foil passed right through it. Only about one in 8,000 was deflected away into the surrounding detecting screen. As a result, Rutherford created a theory that stated that most of an atom was empty space. This made the most sense, since it explained why so few particles were hitting the gold foil. The angle of deflection from the particles also showed that there was most likely a strong positively charged nucleus in the middle of the atom with negatively charged particles circling around it. Rutherford linked this motion to the orbit of planets around the sun. The Bohr atomic model later replaced the Rutherford model.
|

