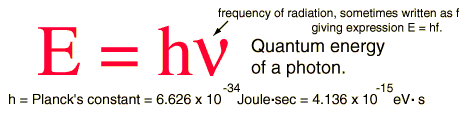|
home | what's new | other sites | contact | about |
|||||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
Professor Jim Al-Khalili, The Mind-Bending Story Of Quantum Physics
In the latter 1800s, Germany was eager to enter the modern industrial age. It spent millions to acquire the patents for Edison’s light bulb. While it was known that a bulb’s filament, charged with electricity, would glow, the underlying physics of how this actually worked was still a big question. In 1887 the German government spent more millions to build a large technical research facility. Political power and international prestige were at stake. Then in 1900, to superintend the research, Max Planck, a 42 year-old scientist, was hired.
why do hot objects glow Planck set himself to discover why the color of the filament changes with the temperature. To put it more generally, why do hot objects glow in the predictable way that they do?
the changing colors of a heated iron poker For many years prior to 1900, German scientists had studied this issue. It had been determined that, as kilowatts increase, a charged object not only gets brighter but the color varies. Think of a hot iron poker. It begins to glow red but then changes to yellow and even white-hot.
light bulbs also exhibit this same sequence of color It was observed that a charged light bulb also begins to glow with shades of red, then to orange, and on to yellow; and, with more charge, the bulb will get hotter and brighter - however, the change in color stops and remains in the yellow-white region.
the blackbody emission problem As we study this area, we come across the term “blackbody” or “Planck’s blackbody” problem. what is a “blackbody” It is not a body colored black, as such, although it could be black. The concept of "blackness" in the term suggests an ability to absorb light; e.g., a dark shirt on a hot summer day will make you feel even warmer because it absorbs more light than it reflects. The term "blackbody" was used in an 1860 scientific paper by German physicist Gustav Kirchhoff...
a perfect blackbody is a theoretical construct and does not exist in nature In the article, Kirchhoff says that a perfect blackbody is a theoretical tool, something that he has "imagined." The perfect blackbody would completely capture all rays of light impinging upon it... Such bodies are called “black” because none of the incident light would escape… Any light emitted from the perfectly absorbing blackbody would come from the heating of the blackbody itself. In this case, the amount of light emitted, Kirchhoff said, would depend on the light-frequency that you see and the temperature of the blackbody. As stated, there is no perfect blackbody, one that completely absorbs all light coming to it. The Sun might come closest to the ideal. Most blackbodies in physics absorb light, more or less, such as a red-hot poker or a glowing light bulb.
the bulb gets hotter and brighter, but why does the color stop changing As the intensity of heat and brightness builds, why don’t we see blue and violet light? - each of which is “hotter” than yellow. There might be a tinge of these, but essentially they're absent. The changing color sequence abruptly halts in the yellow-white range. Why does the trend-line crash when it gets near ultra-violet? This didn’t make sense to the scientists of the day, and it so perplexed them that, in exasperation, they gave it a dramatic name: "the ultra-violet catastrophe"
scientists expected to see a steadily rising slope
Max Planck is called the “father of modern physics,” or the “father of quantum mechanics” - but it was a reluctant paternity, and when the child was born, for more than ten years, he tried to give it back. Planck was not able to solve this conundrum with conventional concepts. Light was a wave, and that was that - everybody “knew” this. It was holy doctrine of physics. However, the concept of “light as wave” did not fit the blackbody-emission research data. What could Planck do? He thought he saw a possible solution, but this answer was heresy. Best not to go there. However, in utter frustration, he allowed himself to posit the unthinkable.
What if light were a particle? - Planck's "act of desperation" When Planck says that “I knew” that this would be of “fundamental significance,” he means “this will be a real game-changer.” When he says “I knew the formula,” he means “I’ve worked out the math, I can see what the answer is, but I don’t like it at all.” And when he says “a theoretical interpretation” must be quickly sought, “no matter how high” the cost, he’s really saying, “If I’m right in all this, then we’re all in trouble, because physics as we’ve known it is done, and so we have to find a grace-saving interpretation to rescue classical Newtonianism from what, sadly, I’ve discovered.”
the genie wouldn’t return to the bottle When Planck redid the math – now armed with a new formula that he invented – suddenly all the numbers magically lined up with the sloping line that crashed into the “ultra-violet catastrophe.” No more upward trendline smoothly going to the Moon: "That's not reality," said the math.
the literary moment of Quantum Mechanic's birth An excerpt from Planck’s 1900 scientific paper:
This "most essential point" is that "E," or energy, light energy, is not smoothly continuous like a wave - at least not with these blackbody emissions - but, in fact, is "continuously divisible ... composed of ... equal parts," that is - light is quantized, light is made up of extremely tiny particles. These are "very definite" in nature, meaning, each is exactly like the next one, each carries the very same energy. This unit-likeness, which Planck worked out mathematically, would later be named after him, the "Planck Constant." However, such fanfare could not cloak his own misgivings that, truly, this was all scientific heresy: "This cannot be! Light is a wave, not a particle!" Unfortunately, for the Newtonian science community, the math could not be strong-armed into proclaiming what convention desired.
"the most revolutionary idea which ever has shaken physics" Max Born (Nobel Prize for Quantum Mechanics in 1954) wrote about Planck: "He was, by nature, a conservative mind; he had nothing of the revolutionary and was thoroughly skeptical about speculations. Yet his belief in the compelling force of logical reasoning from facts was so strong that he did not flinch from announcing the most revolutionary idea which ever has shaken physics."
a very brief look at the math
the famous Planck equation and constant
Planck's constant, an incredibly small number: 6.626 x 10-34 given to us in "joules per second." A joule is a measure of energy. If given “per second,” it becomes a rate, energy transmitted per unit of time. What does this mean? It means many things, but, essentially, though light, or energy, appears to issue as something smooth and unbroken, if we could see the inner structure, we would find a discrete, pixilated version of reality - a latticework of ultra-tiny packets of energy. These individual energy-entities are so small, and so close together, that, to the human eye, it all seems of one piece, and therefore these tiny units must be incredibly small, indeed – but, just how small? Planck’s Constant tells us how small - as small as a photon. That's really small, much smaller than the smallest atom. Editor's note: However, a photon does not have "size" in the ordinary sense of the term. It has neither diameter nor "rest mass" but, as some say, is a conceptual means of energy transference. But let's save this discussion for another time.
Understanding that light is a particle began to solve some of the mystery of blackbody emission - but questions remained. What was the cause of the “ultra-violet catastrophe”? Why did that trend-line crash? Planck and colleagues had no answer for this. Four years later, a junior Swiss patent clerk, third class, would accomplish what the German empire, with its burgeoning coffers and a small army of white labcoats, failed to put right. Studying physics in his spare time and even during breaks at his job, this young office-helper, quietly laboring alone in the laboratory of his own mind, would perceive the missing factor in the decades-old blackbody problem. His name was Albert Einstein.
|
|||||||
|
|