|
home | what's new | other sites | contact | about |
||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
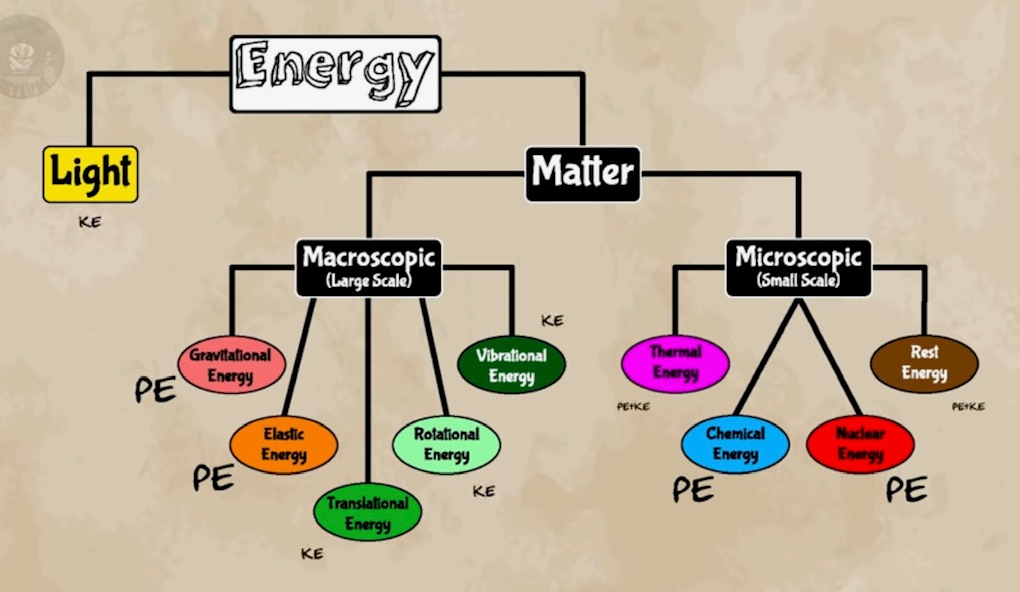
Etymology: ancient Greek for “at work” or (some say) "movement within" Energy gives us the ability to do stuff. Virtually all definitions of “energy” tell us what it does but not what it is. What is it? We could compare and contrast with something we already understand. Or, we could divide it into parts and categorize them. Unfortunately, energy is unlike anything we’ve seen before, and it doesn’t have any parts. If we can find out all the places it can be, then maybe we’ll have a better idea of what it is. We’ve seen stuff near other stuff: we’ll call that potential energy. We’ve seen stuff moving: we’ll call that kinetic energy. We’ve seen stuff being warm: we’ll call that thermal energy. We’ve seen stuff shine: we’ll call that light energy. We’ve seen stuff explode: that’s chemical and nuclear energy. Molecules inside stuff move: that’s just kinetic energy. Molecules have a tendency to stay together but bounce off each other when they get too close: that’s potential energy. Chemical energy is what holds the atoms together in a molecule: that’s potential energy. Nuclear energy holds the atomic nucleus together: that’s potential energy, too.
Everything just seems to be potential energy, kinetic energy, or mass. So, what’s mass? That’s not a simple question to answer fully, but the short version is that it depends on how you look at the stuff in question. If you’re so close that you can see the quarks inside the protons, then it doesn’t look like there’s a whole lot of mass there. But if we zoom out, it all just looks like a single proton. All that potential energy looks like mass because we can’t see it anymore. The point is almost all the energy we call “mass” is just the potential energy inside the nuclei of the atoms. You’re most just empty space. So, in the end, all energy is really just potential energy and kinetic energy. Some of it just happens to be in the nooks and crannies inside things. Textbooks say that energy (physical) is the capacity to do work. “Energy” and “work” are properties. A rolling ball -- we can label where it is by assigning a property of “position”; we label its motion by assigning a property of “velocity” An object can have motion and that motion is real, but “velocity” is not real, just a useful number we assign to it. Properties are useful because they measure something we want to know. Properties -- Measure What? Position --- location Velocity --- motion Acceleration --- change in motion Force --- influence, interaction Energy --- ? Work --- ? Work (physical) = done on an object by a force, as long as that force contributes in some way to how the object moves, i.e., the object’s displacement. Push on a book with enough force to make it move across a table = “work” done on the book Work = force x displacement The key here is displacement. In order for “work” to be done on that book, it has to move from one place to another. If the object doesn’t go anywhere, then no “work” has been done. Stuff has to happen for “work” to be done. Work (physical) is the amount of stuff that has happened; or, if you’re making predictions, the amount of stuff that will happen. So, what does this make “energy”? If “energy” is the capacity to do work, and “work” is the amount of stuff that has or will happen, then energy is the amount of stuff that could, potentially, happen. Energy = work = stuff that could happen There’s an upper limit to the amount of stuff that could happen, and that upper limit is measured as energy. Stuff: lifting, swimming, climbing, dancing, waving, running, slithering, boiling, electrifying, chemically reacting, nuclear reacting… These are all examples of energy being used to do “work”. If you’re lifting a box, you’re turning chemical energy in your body into gravitational (potential) energy. That change “works” on the box, moving it upward. You didn’t use all the energy inside your body to do that work, just a very small amount. All the energy in your body = all the stuff that you could possibly do. The work is what you actually do. That box you lifted, is now infused with energy to do stuff – like fall down. That gravitational energy is gradually transformed into kinetic energy on the way down. But that’s not all the energy in that box. In addition to gravitational and kinetic energy, it also has many other kinds of energy, tied up in the material – none of which are being used to do “work”. The only “work” done is the box falling, that’s the stuff that’s “happening”. So, how do we measure these things? We don’t – that is, we can’t measure them directly. Unlike position and velocity, energy and work are properties that are hidden from view. We have to calculate their value by measuring something else. If it’s gravitational (potential) energy, we have to measure a distance from some reference location, usually, the ground, and then calculate the energy using some equation. “Twice as high” means “twice the energy,” meaning it could do twice as much stuff. If it’s kinetic energy, we calculate it with a measured speed. “Twice the speed” means “four times the energy” or four times as much stuff. There are equations for finding all types of energy, and every one adds to what an object could possibly do. If you can change the energy from one type to another, or move it from one object to another, then you’re doing “work”. “Something” will be happening. There are a lot of different places we can find energy, so it helps to arrange them into categories. We could take the list and organize them according to where we find them… But there’s something we can do that’s more useful. All energies can be described by two generic types: Potential energy, related to position or location Kinetic energy related to motion That’s all there really is. Remember, the purpose of energy is show us what could happen. And things happen only if they move from one place to another. Location and motion pretty much cover that. So, you could label all of these various kinds of energy as either potential or kinetic energy: nuclear energy is just the potential energy of protons and neutrons. Chemical energy is just the potential energy in chemical bonds. Thermal energy is just the wiggly kinetic energy plus some material potential energy. But don’t location and motion depend on your point of view? Yes, and that’s just more evidence that energy is not something tangible. Energy is not a “thing” by itself, it’s a property of things. So, there’s no reason in particular we’d all have to agree on how much is there. If location and motion are relative to the observer, then so are potential and kinetic energy. There’s always some kind of reference point where you define zero. For kinetic energy, it’s usually your own speed. Two observers, moving at different speeds, aren’t going to agree on the motion of a third object. And so they’re not going to agree on its energy, either. That’s fine, because energy is not a tangible thing, it’s just a number.
And a similar thing happens with potential energy. For gravitational energy, the reference is usually the ground. But there’s nothing special about that choice, you could move that reference around (higher or lower), and it’s totally fine. What matters is the difference in energy between two events across time. That’s the “work” done. That’s what’s actually happening. So, what is energy? Energy is the amount of stuff that could happen. In the end, all that really matters is what does happen. And that takes ”work”. But these definitions just tell us what energy does. We don’t really know what energy is per se. Every hypothesis we’ve ever had that has pretended that it’s some kind of substance has epically failed. It seems to be an abstract property of the universe. Energy is just a number, and the total amount never changes.
|
||||
|
|