|
home | what's new | other sites | contact | about |
|||||||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
Harvey Fletcher (1884 - 1981) & Robert Millikan (1868 - 1953)
Wikipedia: Harvey Fletcher (September 11, 1884 – July 23, 1981) was an American physicist. Known as the "father of stereophonic sound", he is credited with the invention of the 2-A audiometer and an early electronic hearing aid. He was an investigator into the nature of speech and hearing, and made contributions in acoustics, electrical engineering, speech, medicine, music, atomic physics, sound pictures, and education. In 1911, Fletcher was the first physics student to earn a Ph.D. summa cum laude from the University of Chicago. His dissertation research was on methods to determine the charge of an electron. This included the oil drop experiment commonly attributed to his advisor and collaborator, Robert Andrews Millikan. Millikan took sole credit, in return for Fletcher claiming full authorship on a related result for his dissertation. Fletcher's contributions were detail-oriented but still contributed to the successful experiment, in which he incorporated, among other things, experience with projection lanterns. Millikan went on to win the 1923 Nobel Prize for Physics, in part for this work, and Fletcher kept the agreement a secret until his death.
Wikipedia: Robert Andrews Millikan (March 22, 1868 – December 19, 1953) was an American experimental physicist honored with the Nobel Prize for Physics in 1923 for the measurement of the elementary electric charge and for his work on the photoelectric effect. Starting in 1908, while a professor at the University of Chicago, Millikan, with the significant input of Fletcher … and after improving his setup, published his seminal study in 1913. This remains controversial since papers found after Fletcher's death describe events in which Millikan coerced Fletcher into relinquishing authorship as a condition for receiving his PhD. In return, Millikan used his influence in support of Fletcher's career at Bell Labs... A religious man and the son of a minister, in his later life Millikan argued strongly for a complementary relationship between Christian faith and science. He dealt with this in his Terry Lectures at Yale in 1926–27, published as Evolution in Science and Religion. He was a Christian theist and proponent of theistic evolution. A more controversial belief of his was eugenics – he was one of the initial trustees of the Human Betterment Foundation and praised San Marino, California for being "the westernmost outpost of Nordic civilization ... [with] a population which is twice as Anglo-Saxon as that existing in New York, Chicago, or any of the great cities of this country." In 1936, Millikan advised the president of Duke University in the then racial segregated southern United States against recruiting a female physicist and argued that it would be better to hire young men. On account of Millikan's affiliation with the Human Betterment Foundation, in January 2021, the Caltech Board of Trustees authorized removal of Millikan's name … from campus buildings.
one of the most ingenious experiments in the history of science The “oil drop” experiment measured the charge of a single electron. One electron. But how would you do that? We now know that an electron is nearly 2000 times smaller than the smallest atom – hydrogen. The electron is so small that if a nucleus were the size of baseball, the electron would be a mile away. That’s small. How could such a small entity as an electron be measured?
a scene from a Superman movie offers a clue In “Superman Returns” (2006) we find a young Clark Kent learning to fly. He loses control, though, starts to fall, and crashes through the roof of his father’s barn:
However, his descent is suddenly interrupted, the force of gravity is overcome, and Clark finds himself…
hovering, suspended in mid air, as if weightless
this is what happened to some of Fletcher and Millikan’s falling oil drops They entered a state of levitation and abeyance. How did this happen? Gravity was still in play, hadn't gone away – but an opposing, exactly counterbalancing, force stopped the action cold. We’ll explore this. two opposing forces, gravity and electricity As we’ve seen, J.J. Thompson determined that atoms have electrons and these are negatively charged. But he wasn’t able to quantify the charge. Therefore, the question remained, just how much charge does an electron possess? But, how do go about the evaluation? You can’t just pull out a single electron, put a rule up against it or attach wires. In one of the most clever experiments ever devised, Fletcher and Millikan put a specific number to the charge of an electron. They did this by suspending falling oil drops in mid air via two opposing forces, gravity and electricity.
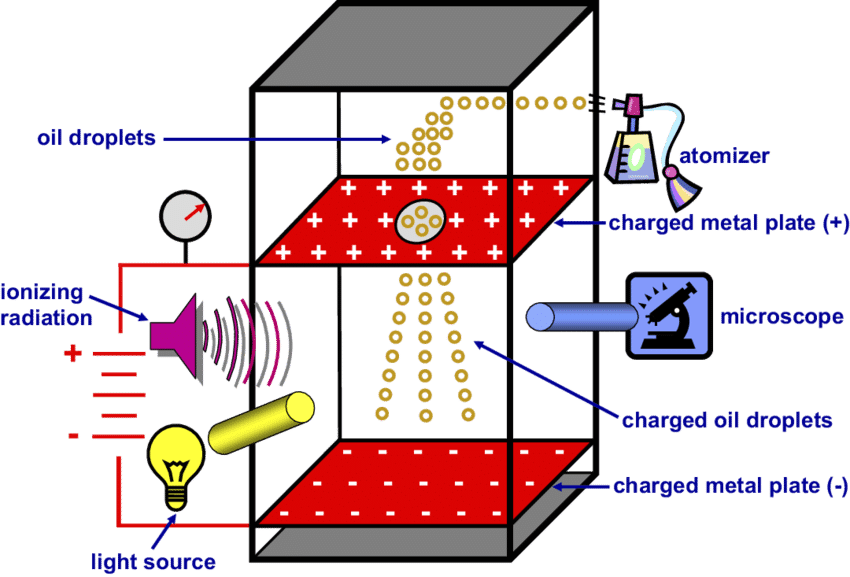
Here’s a stylized version of the Fletcher-Millikan apparatus. Think of a sealed chamber – the diagram gives us a cut-away view. On the upper right, see the “atomizer,” which is like a perfume mister, spraying very tiny droplets of oil. Notice the two red metal plates. The one on the top is given a positive charge and the plate on the bottom is negatively charged. We also take note that there’s a very tiny hole in the top plate, allowing some of the oil-mist droplets to enter the chamber, which is situated between the two metal plates. Also, on the right side of the chamber is a microscope. The mist-droplets are so small that a microscope is needed to observe them. through the microscope, what does one see A drop or drops of oil, proceeding through the tiny hole in the top metal plate, would fall into the middle chamber. Here, with aid of the microscope, you would see the drop(s) moving down, under the force of gravity.
Keep in mind that the falling drop, now in the central chamber of the apparatus, is between two metal plates. If the plates become electrically charged, a new force will act upon the droplet.
Here we see the electrical force counterbalancing the gravitational force.
like charges repel Notice, too, that Dr. DeWitt has a plus sign (“+”) on the oil droplet. This means that the oil droplet is positively charged. Therefore, because like charges repel, as the droplet moves downward toward the bottom positively-charge plate, the droplet will be pushed away, repelled in an upward direction, by the bottom plate. In this experiment, we can control the amount of force generated by the two metal plates; which is to say, the voltage can be controlled. Voltage is a kind of pressure the pushes charged electrons (current). See the upper diagram with the two red metal plates. There, we find a dial:
This dial indicates that we can control the voltage to the metal plates. If we crank up the voltage, there'll be more "pressure" from the lower plate, and the oil droplet will overcome the force of gravity and will rise toward the top of the chamber:
However, if we move the dial the other way and tone down the voltage, then the electrical force will be smaller than the gravitational force, and so the droplet will sink toward the bottom.
the Goldilocks setting And so, what Fletcher and Millikan were looking for was the “Goldilocks setting,” where the voltage was “just right” relative to the gravitational force. With this equilibrium, the droplet would achieve a kind of stasis, perfectly balanced in mid air.
What is gained by achieving this balancing of forces? A great deal – because, if the forces are balanced, if both sides are equal, well then, now we have the opportunity to express these equal parts as a mathematical equation. electric force = gravity force When the drop hangs motionless in mid-air, this means that the electric force is equal to the gravitational force. What are the components of each force?
we find 3 quantities in question: The force of gravity depends on (1) the mass of the oil drop. The force of electricity depends on (2) the electrical charge of the drop and (3) the voltage on the plates.
2 of the 3 were readily determined Fletcher and Millikan were readily able to determine (1) the mass of a drop and (2) the voltage on the plates, and so the only remaining issue was the quantification of (3) the charge of the drop. Let's remind ourselves of the purpose of this entire exercise: Fletcher and Millikan were trying to determine the exact electrical charge for one electron. simple algebra They now have the beginnings of an equation: electrical force = gravitational force. Further, they already know the values of two of the three items in question. Armed with this information, they can now, with simple algebra, "solve for the unknown." What is the unknown? - the charge on the drops, which would lead to an understanding of the charge of an individual electron. here's what happened next They observed a great many oil drops. The drops varied somewhat in size. The larger drops required a little more voltage to achieve stasis. And larger drops also had a larger electrical charge. They made a list of all the data. And, as they looked at the data, they noticed that the charge on the drops came in exact whole-number multiples of "1.6". Editor's note: This "1.6" could be expressed as "16" or "160" etc., depending on the unit of expression adopted, the particular fraction of "coulomb" [a measure of charge] chosen, but let's stay with "1.6" for our purposes here. whole-number multiples of 1.6 Some of the drops had a charge of 1.6; others had a charge of 3.2 (2 x 1.6); and others had a charge of 4.8 (3 x 1.6), etc. There was no "1.6 and a half" or any other fractional answer. In other words, the oil droplets were gaining or losing charge in precise whole-number units of 1.6. From this observation, Fletcher and Millikan determined (within 1% of the currently accepted value) that the smallest unit-charge of an electron is: 1.602176634 × 10-19 coulomb In other words, the charge of an electron is “quantized.” It comes in definite, discrete, unvarying units of energy. Editor's note: see the "definitions" page for more on "quanta."
Britannica: "In addition to the electron, all freely existing charged subatomic particles thus far discovered have an electric charge equal to this value or some whole-number multiple of it." it's called 'the elementary charge' Wikipedia: "The elementary charge, usually denoted by e or sometimes q? is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 e. This elementary charge is a fundamental physical constant."
Footnote: the bottom metal plate in Dr. DeWitt’s example is positively charged but many textbooks have the bottom plate negatively charged Our original diagram near the top of the page has the bottom plate negatively charged.
I checked a few sources and the answer seems to be this: The experiment will work with either a positive or negative bottom plate if the oil drops have their charges adjusted accordingly. Recall that "like charges repel" and that, if the bottom plate is negatively charged, then the oil drop will need to be of the same charge for it to move upwards (and vice versa). Look at the left side of the diagram for the “ionizing radiation.” This was done with x-rays which created ions.
X-rays, it seems, can create, directly or indirectly, either cations or anions concerning the atoms of the oil drops. This happens when the high-energy x-rays “kick out” electrons (1) in the atoms of gases within the central chamber or (2) of the atoms in the oil drop itself. If (1) occurs, then these “free” electrons might attach to the atoms of an oil drop rendering it negatively charged; or, (2) if the x-rays dislodge electrons in an oil drop, then the drop will become positively charged.
|
|||||||||
|
|