|
home | what's new | other sites | contact | about |
||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
website: https://seatofthesoul.com
Editor’s note: “Wu Li” is the Chinese word for physics; literally, “patterns of organic energy”
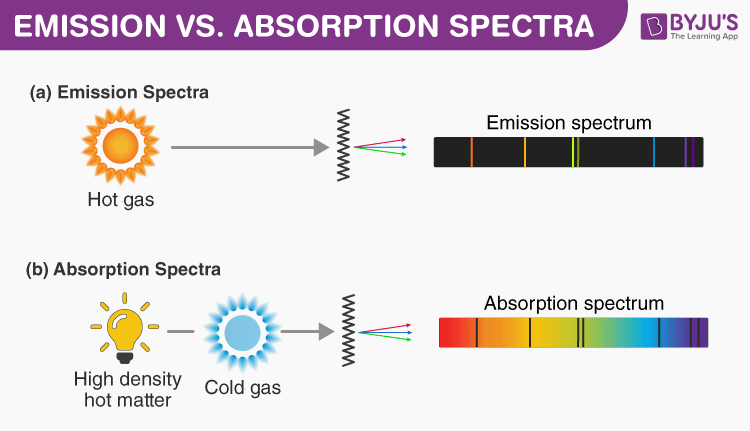
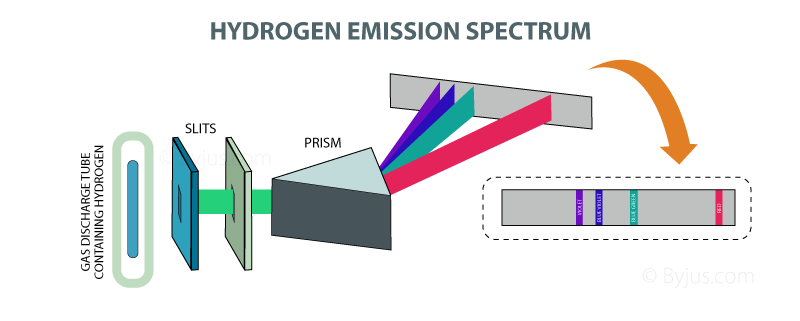
Excerpts from Gary Zukav's book: According to Bohr s theory, the electron in the hydrogen atom stays as close to the nucleus as it can get. In other words, it usually is in the first shell. This is the lowest energy state of a hydrogen atom. (Physicists call the lowest energy state of any atom its ground state.) If we excite an atom of hydrogen we cause its electron to jump to one of the outer shells. How far it jumps depends upon how much energy we give it. If we really heat the atom up (thermal energy), we cause its electron to make a very large jump, all the way to one of the outer shells. Smaller amounts of energy make the electron jump less far. However, as soon as it can (when we stop heating it), the electron returns to a shell closer in. Eventually, it returns all the way back to shell number one. Whenever the electron jumps from an outer shell to an inner shell, it emits energy in the form of light. The energy that the electron emits is exactly the amount of energy that it absorbed when it jumped outward in the first place. Bohr discovered that all of the possible combinations of jumps that the hydrogen electron can make on its journeys back to the ground state (the first shell) equals the number of lines in the hydrogen spectrum… If the electron in a hydrogen atom travels from an outer shell all the way to the innermost shell in one jump, it gives off a certain amount of energy. That makes one line in the hydrogen spectrum. If the electron in a hydrogen atom makes a tiny jump from an outer shell to the next shell inward, it gives off a much smaller amount of energy. That makes another spectral line. If the electron in a hydrogen atom jumps from shell five to shell three, for example, that makes yet another line. A jump from shell six to shell four, and then from shell four to shell one, makes two more spectral lines, and so on. In this way, we can account for the entire hydrogen spectrum. If we excite a hydrogen atom with white light instead of heat, we can produce the absorption phenomenon… Each electron jump from an inner shell to a shell farther out requires a certain amount of energy, no more and no less. An electron jump from shell one to shell two requires a certain amount of energy, and only that amount. The same is true for a jump from shell five to shell seven, etc. Each jump that the electron makes from an inner shell to an outer shell takes a specific amount of energy, no more and no less. When we shine white light on a hydrogen atom, we are offering it a whole supermarket of different energy amounts. However, it cannot use all that we have to offer, only certain specific amounts. If its electron jumps from shell one to shell four, for example, it takes that particular energy package out of the array of energy packets that we are giving it. The package that it takes out becomes a black line in the otherwise complete spectrum of white light. A jump from shell three to shell four becomes another black line. A jump from shell one to shell two, and then from shell two to shell six (there are all sorts of combinations), makes two more black lines. In sum, if we shine white light through hydrogen gas and then through a prism, the result is the familiar white-light spectrum, but with over one hundred black lines in it. Each of these black lines corresponds to a specific energy amount that was required to make a hydrogen electron jump from one shell to another shell farther out…
Each colored line in the hydrogen spectrum is made from the light emitted from hydrogen electrons as they jump from a particular outer shell to a particular inner shell. What we did not mention earlier is that some of the lines in the hydrogen spectrum are more pronounced than others. The lines that are more pronounced are always more pronounced and the lines that are faint are always faint. The intensity of the lines in the hydrogen spectrum varies because hydrogen electrons returning to the ground state do not always take the same route.
Shell five, for example may be a more popular stopover than shell three. In that case, the spectrum produced by millions of excited hydrogen atoms will show a more pronounced spectral line corresponding to electron jumps from shell five to shell one and a less pronounced spectral line corresponding to electron jumps from, say, shell three to shell one. That is because, in this example, more electrons stop over at shell five before jumping to shell one than stop over at shell three before jumping to shell one. In other words, the probability is very high, in this example, that the electrons of excited hydrogen atoms will stop at shell five on their way back to shell one and the probability is lower that they will stop at shell three. Said another way, we know that a certain number of electrons probably will stop at shell five and that a certain lesser number of electrons probably will stop at shell three...
|
||||
|
|