|
home | what's new | other sites | contact | about |
||||||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
how to win a quantum teddy-bear at the county fair Professor Jim Al-Khalili offered this analogy: Let’s say you’re at the county fair, and you need to knock down all the tin cans to win a teddy. Can you do it? You're offered two different kinds of balls, red and blue, to throw at the cans.
The red balls are very lightweight, puffballs, hollow plastic balls for a small child. The blue balls are dense and heavy golf balls. If we throw the red balls, even a handful of them, at the cans, and we hit them, there’ll be no effect. There’s just not enough energy carried by the red balls. But if we pitch the blue golf balls, now the stack of cans shatters easily.
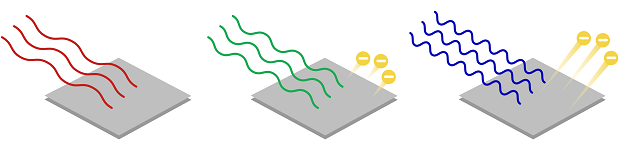
the photoelectric effect is sort of like knocking down a stack of cans – but don’t try it with red light, you have to use blue or violet light What is the “photoelectric effect”? Imagine a piece of metal. There are electrons as part of the metal. These electrons are like the stack of cans. Now let’s shine a light on the metal.
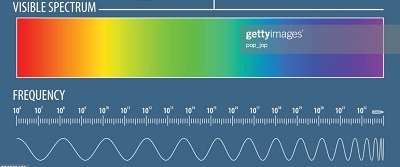
“photoelectric” means “photon/light” and “electrons” If we use a red light, light with low frequency, low energy, the electrons on the surface of the metal remain undisturbed. This is like the red puffballs and the cans. But if we shine a blue or violet light, light with higher frequency, higher energy, some of the electrons of the metal will be set free and ejected. This is like the blue golf balls leveling the stack of cans.
Einstein read Planck’s paper, and also Lenard's research concerning his "photoelectric" experiment, all of which led to a radical new idea In general outline, Einstein’s coming-to-insight in 1905 seems to have been something like this: “Lenard's photoelectric experiments cannot be adequately explained if we cling to the classical concept of light as a wave. There’s not enough energy to dislodge the electrons with light as a wave. But what if light is not a wave? – or, at least, not a wave all the time. What if it’s a particle? There is enough energy to dislodge electrons if we accept that light is a particle. Well, Planck suggested that it’s a particle. But he was reluctant to accept his own findings. Planck is the 'unwilling convert' and talks as if light-as-particle were just some kind of gimmick to make the math work out, on our way to finding a new interpretation to support the old classical view. But what if it’s really true, not just a math gimmick but, what if light really is a particle? What if we need a whole new paradigm for physics, a whole new way of looking at the world and the universe?”
Planck reluctantly hinted at the need to reconstruct physics, but the true beginning of Quantum Mechanics began with the conviction of Einstein concerning his new insight -- light is a particle, a "quantum" Einstein knew that he was playing with revolutionary scientific ideas. See this letter to a friend and the highlighted area:
"the most revolutionary sentence written by a physicist of the twentieth century" Some reviewers have offered the above assessment. In Einstein's paper on the photoelectric effect, he speaks of light as both particle and wave. This had never before been proposed. Notice, too, how he refers to "energy quanta." In 1921 Einstein would win a Nobel Prize – not for Relativity but for the quantum implications of the Photoelectric Effect
postscript: an answer to the "ultra-violet catastrophe" The new research in the area of the photoelectric effect would also provide further answer concerning the old blackbody issue. Professor Jim Al-Khalili had this to say: “Einstein’s new idea also helped solve Planck’s mystery of the light bulb [and the ultra-violet catastrophe]. There was more ‘red’ [in the light bulb glow] than ultra-violet quanta because ultra-violet took so much more energy to create – about a hundred times more energy. No wonder there were so few of them.” Let’s recall the upwardly sloping line to “infinity”:
Scientists of the classical era had theorized that blackbody energy levels might rise, on and on, exponentially. They viewed light as a steady, unbroken stream of energy; however, the reality revealed energy in terms of "quanta," tiny packets of energy in precise -- not unlimited -- amount. Energy doesn’t increase exponentially, and indefinitely. The system doesn’t work that way. The blackbody emission downward slope suggests that, while energy increases for a time, in a limited way, you can’t just keep on multiplying and multiplying it. It takes too much energy to keep that line continually moving upward: the “circuits become overloaded,” we’re “overdrawn at the bank,” and we “hit the wall” at the ultra-violet barrier.
|
||||||||
|
|