|
home | what's new | other sites | contact | about |
||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
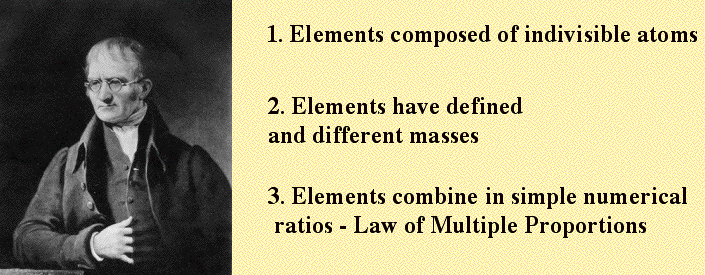
John Dalton (1766 - 1844)
While Democritus’ idea of “the uncuttable” was not unknown in the 1800s, there was no real substantiation for it. British scientist John Dalton, in 1808, was the first to provide experimentation offering good evidence that matter is composed of tiny atoms.
How did Dalton accomplish this without high-tech equipment? He demonstrated that compounds are produced when elements combine, but always in the same ratio of quantity by mass. It’s called the “law of multiple proportions.” For example, 1 atom of oxygen combines with 2 atoms of hydrogen to form a water molecule, H2O. However, oxygen can also combine with hydrogen on a 2 by 2 basis, 2 atoms of oxygen combine with 2 atoms of hydrogen to produce hydrogen peroxide, H2O2. always in whole numbers There are many examples of two elements combining to form different compounds, but, when they do, Dalton noticed, it always occurs in ratios of whole numbers. In other words, oxygen does not combine with half an atom of hydrogen, or some other fraction. Dalton surmised that an atom, in essence, is an unchanging, indivisible entity which unites with other atoms in predictable manner, in whole-entity units, and with specific ratio combination. Reasonings by Dalton such as this served as basis of the first experiments which gave evidence of the existence of atoms.
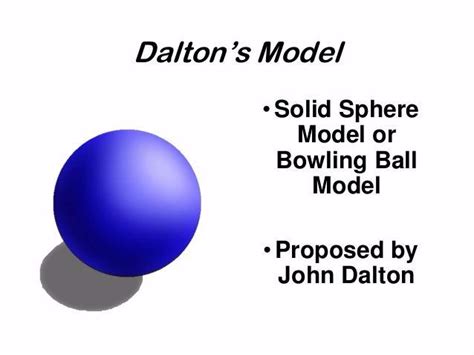
Dalton’s “solid sphere” or “bowling ball” model of the atom was a good start. But others, later in the nineteenth century, would begin to modify this image.
|
||
|
|