|
home | what's new | other sites | contact | about |
||||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Quantum Mechanics
return to "Quantum Mechanics" main-page
Niels Bohr (1885 - 1962)
Wikipedia: Niels Henrik David Bohr (7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Bohr was also a philosopher and a promoter of scientific research. Bohr developed the Bohr model of the atom, in which he proposed that energy levels of electrons are discrete and that the electrons revolve in stable orbits around the atomic nucleus but can jump from one energy level (or orbit) to another. Although the Bohr model has been supplanted by other models, its underlying principles remain valid. He conceived the principle of complementarity: that items could be separately analysed in terms of contradictory properties, like behaving as a wave or a stream of particles. The notion of complementarity dominated Bohr's thinking in both science and philosophy. Bohr founded the Institute of Theoretical Physics at the University of Copenhagen, now known as the Niels Bohr Institute, which opened in 1920. Bohr mentored and collaborated with physicists including Hans Kramers, Oskar Klein, George de Hevesy, and Werner Heisenberg. He predicted the existence of a new zirconium-like element, which was named hafnium, after the Latin name for Copenhagen, where it was discovered. Later, the element bohrium was named after him.
from pediaa.com
from lisbdnet.com
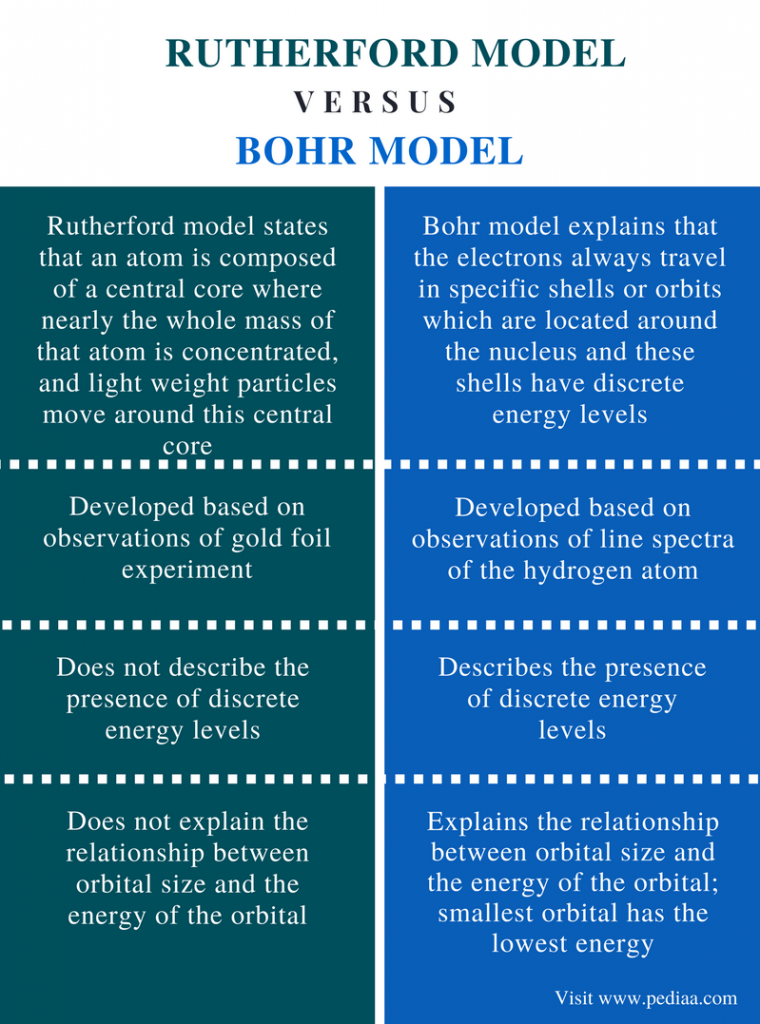
How Does Bohr’s Model Of The Atom Differ From Rutherford’s?Rutherford described the atom as consisting of a tiny positive mass surrounded by a cloud of negative electrons. Bohr thought that electrons orbited the nucleus in quantised orbits. … He believed that electrons moved around the nucleus in circular orbits with quantised potential and kinetic energies. How did Bohr’s model differ from Rutherford’s quizlet?Bohr’s model states that electrons occupy orbitals with fixed energy levels. … Rutherford’s model explained the physical and chemical properties of elements, whereas Bohr’s model only explained the physical properties... Bohr’s model proposed that electron orbitals have different shapes. What did Bohr’s model have that Rutherford’s didn't?The atom is mostly empty space. Electrons orbit around the center of the atom. The atom’s positive charge is located in the atom’s nucleus. What is the main difference between Bohr’s model of the atom and the atomic theory that is currently accepted?Bohr assumed that electrons traveled in orbits around the nucleus. Current atomic theory assumes electrons do not travel in fixed paths. How was Bohr’s model different?Bohr’s model shows that electrons travel in specific orbits around the nucleus which differs from Rutherford’s model that says the electrons are spread out in a cloud around the nucleus.
How is Bohr’s model different from previous models?Unlike earlier models, the Bohr Model explains the Rydberg formula for the spectral emission lines of atomic hydrogen. The Bohr Model is a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus similar to the planets orbiting the sun (except that the orbits are not planar).
What did the Bohr model of the atom include?The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). Bohr used the term energy levels (or shells) to describe these orbits of differing energy. … The energy level an electron normally occupies is called its ground state.
What did the Bohr model of the atom add?Bohr’s atomic model [is based on] hydrogen emission spectra. Bohr explained that electrons can be moved into different orbits with the addition of energy. When the energy is removed, the electrons return back to their ground state, emitting a corresponding amount of energy – a quantum of light, or photon. What did Rutherford’s model of the atom include that Thomson's didn't?Thomson’s plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged “soup.” Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus.
What is the difference between an energy level such as in the Bohr model and the actual electron cloud?Bohr’s model treats electron energy levels as clearly defined orbital paths around the nucleus (like planets orbit the Sun). The cloud model treats the energy levels as probability clouds, i.e. regions in which electrons are likely to be found. What is the main difference between the cloud model of the atom and the modern model?The main difference between the two models was the placement of the electron in an atom. What did Rutherford’s model and Bohr’s model have in common?Bohr’s model is a defined, expanded model of Rutherford’s atom that overcomes these two drawbacks. The basics are the same, i.e., electrons revolve around the nucleus in paths called orbits with the nucleus at the centre. Bohr expanded on Rutherford’s model in detail. What does the Bohr model contribute to the theory on the structure of the atom?Electrons present in the orbits closer to the nucleus have larger amounts of energy. Niels Bohr proposed that electrons circled the nucleus of an atom in a planetary-like motion. Bohr’s theory explained the line spectra of the hydrogen atom. According to the Bohr model of atoms, electrons occupy definite orbits. In what way is Bohr’s model of atom similar to Rutherford’s model of atom?1) Both of them said that the electrons revolve around the nucleus. 2) They both agreed that the electron occupies a large space of the atom. In fact, Bohr didn’t proved Rutherford’s model wrong but he expanded and improved his model of the atom. What did Bohr’s model of the atom include that Rutherford’s model did not have?Bohr’s work with atomic spectra led him to say that the electrons were limited to existing in certain energy levels, like standing on the rungs of a ladder. What did Bohr’s model of the atom include that Rutherford’s model did not have? … The atom is mostly empty space. Electrons orbit around the center of the atom. What did the Bohr model explain?In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. … The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another.
What does the Bohr model represent?The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. How does Bohr’s model explain atomic spectra?Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed. … This produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of an element.
How did Bohr expand on Rutherford’s model of the atom?To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. … The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. How did the Bohr model describe electrons within an atom and how did this model explain line spectra?Bohr’s model explains the spectral lines of the hydrogen atomic emission spectrum. While the electron of the atom remains in the ground state, its energy is unchanged. When the atom absorbs one or more quanta of energy, the electron moves from the ground state orbit to an excited state orbit that is further away. Why is the Bohr model important?The Bohr model is important because it was the first model to postulate the quantization of electron orbits in atoms. Thus, it represents an early quantum theory that gave a start to developing modern quantum theory. It introduced the concept of a quantum number to describe atomic states. What did Bohr discover about the atom?The Bohr model shows the atom as a small, positively charged nucleus surrounded by orbiting electrons. Bohr was the first to discover that electrons travel in separate orbits around the nucleus and that the number of electrons in the outer orbit determines the properties of an element.
Why was Bohr’s model inaccurate?First, the Bohr model violates the Heisenberg Uncertainty Principle, since it states that electrons have a known radius and orbit. The Bohr Model also provides an incorrect value for the ground state orbital angular momentum and doesn’t work as well for creating diagrams of larger atoms. When did Bohr propose his model of the atom?In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values. How did Rutherford’s experimental evidence lead to the development of a new atomic model?Rutherford’s experiment prompted a change in the atomic model. If the positive alpha particles mostly passed through the foil, but some bounced back. AND if they already knew that the electron was small and negative, then the atom must have a small positive nucleus with the electrons around them. What change to the atomic model helped solve the problem in Rutherford’s model?Bohr’s work with atomic spectra led him to say that the electrons were limited to existing in certain energy levels, like standing on the rungs of a ladder.
How Bohr explain stability of atom?Niels Bohr explained the stability of the atoms through the concept of revolution of electrons in different energy levels. The change in the energy of an electron occurs when it jumps from a lower energy level to a higher energy level or vice versa. … In this way, energy is not lost and the atom remains stable.
How does the Bohr model explain that each element has a unique emission spectrum?In the Bohr model, electrons can exist only in certain energy levels surrounding the atom. When electrons jump from a higher energy level to a lower one, they emit light at a wavelength that corresponds to the energy difference between the levels. The energy levels in each atom are unique. How does Bohr’s concept of an energy level differ from Schrodinger’s?In the Bohr model, the electrons are particles that occupy only certain orbits of fixed energy around the nucleus. In the Schrödinger model, the electrons behave as standing waves that have greater probability of being in some regions of space (orbitals) than in others.
How do electrons in the same atom differ?The central structure of an atom is the nucleus, which contains protons and neutrons. This nucleus is surrounded by electrons. Although these electrons all have the same charge and the same mass, each electron in an atom has a different amount of energy. … Electrons that have higher energy are found further away. Is Bohr’s model of the atom correct?This model was proposed by Niels Bohr in 1915; it is not completely correct, but it has many features that are approximately correct and it is sufficient for much of our discussion. How was the Bohr model disproved?Five years later, the model would be disproved by Hans Geiger and Ernest Marsden, who conducted a series of experiments using alpha particles and gold foil – aka. the “gold foil experiment.” In this experiment, Geiger and Marsden measured the scattering pattern of the alpha particles with a fluorescent screen. What makes it difficult to represent the model of an atom?Atoms are much larger than their nuclei. Electrons are too small to be shown to scale. Electrons are constantly in motion and do not truly follow orbital paths.
|
||||
|
|